Butylated Hydroxyanisole-Induced Alterations in the Stomach and Kidney of Albino Rat : A Mini Review
DOI:
https://doi.org/10.26438/ijsrbs.v12i3.685Keywords:
Butylated hydroxyanisole, stomach, kidney, toxicityAbstract
Butylated hydroxyanisole or commonly abbreviated as BHA, is mostly employed as a food preservative to prolong the products shelf life by stopping or postponing oxidation processes. The commercial product is usually a combination of 90% 3-BHA and 10% 2-BHA. BHA is generally considered safe when used as a food additive, Since, butylated hydroxyanisole is so widely used, it can be detected in both human tissues and a variety of environmental matrices. Humans are mostly exposed to butylated hydroxyanisole through their diet. One of the primary metabolites that butylated hydroxyanisole can produce under different conditions is tert-butyl hydroquinone (TBHQ). According to a number of studies, butylated hydroxyanisole may harm the thyroid system and result in growth and metabolic problems, neurotoxicity, and cancer. One of the main priorities is to minimize the harmful effects of BHA. Future studies should concentrate on identifying safe, non-toxic, and eco-friendly substitutes for BHA. This review aims to highlight the hazardous and detrimental effects of butylated hydroxyanisole in animal models. We are confident that this assessment will yield important data about the toxicological nature of butylated hydroxyanisole, that will aid in the development of safe usage guidelines.
References
P.B. Jean. “Food Preservation,” Nicolas Appert inventeur at humaniste, 2-908670-17-8, 1994.
G. Rychen, G. Aquilina, G. Azimonti, V. Bampidis, M. Bastos , G. Bories, A. Chesson, P. S. Cocconcelli, G. Flachowsky, B. Kolar, M. Kouba, M. L. Alonso, S. L. Puente, A. Mantovani, B. Mayo, F. Ramos, M. Saarela, R. E. Villa, R. J. Wallace, P. Wester, Anne‐Katrine Lundebye, C. Nebbia, D. Renshaw, M. L. Innocenti, J. Gropp, “Safety and efficacy of butylated hydroxyanisole (BHA) as a feed additive for all animal species,” European Food Safety Authority Journal (EFSA), Vol. 16, no. 3, pp.e05215, 2018.
F. Shahidi and P. Ambigaipalan, “Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects – A review”, Journal of Functional Foods, Vol.18, pp. 820–897, 2015.
A. Makahleh, B. Saad and M.F. Bari, “Synthetic phenolics as antioxidants for food preservation”, in Handbook of Antioxidants for Food Preservation, Cambridge, U.K. : Woodhead Publishing, pp. 51–78, 2015.
F. Aguilar, R. Crebelli, B. Dusemund, P. Galtier, J. Gilbert, D.M. Gott, U. G. Remi, J. Koenig, C. Lambré, J-C. Leblanc, A. Mortensen, P. Mossesso, D. Parent-Massin, I.M.C.M. Rietjens, I. Stankovic, P. Tobback, I. Waalkens-Berendsen, R.A. Woutersen and M. Wright, “Scientific opinion on the re-evaluation of butylated hydroxyanisole- BHA (E 320) as a food additive”, European Food Safety Authority Journal (EFSA),Vol. 9, pp. 2392, 2011.
R. Rodil , J.B. Quintana, G. Basaglia, M.C. Pietrogrande and R.Cela, “Determination of synthetic phenolic antioxidants and their metabolites in water samples by downscaled solid-phase extraction, silylation and gas chromatography-mass spectrometry”, Journal of Chromatography A, 1217, pp. 6428–6435, 2010.
C. André, I. Castanheira, J. M. Cruz, P. Paseiro, and A. Sanches-Silva, “Analytical strategies to evaluate antioxidants in food: a review,” Trends in Food Science & Technology, Vol. 21, pp. 229–246, 2010.
P. G. Demertzis and R. Franz, “Development of an HPLC method for measurements of the stability of Irganox-type polymer antioxidants in fatty food simulants,” Z. Lebensmittelunters. Forsch. A, Vol. 206, pp. 193–198, 1998.
H. Verhagen, P. A. Schilderman, and J. C. Kleinjans, “Butylated hydroxyanisole in perspective,” Chemico-. Biological Interaction., Vol. 80, pp. 109–134, 1991.
R. Liu and S. A. Mabury, “Synthetic phenolic antioxidants and transformation products in dust from different indoor environments in Toronto, Canada,” Science of the Total Environment, Vol. 672, pp. 23–29, 2019.
R. Liu, Y. Lin, T. Ruan, and G. Jiang, “Occurrence of synthetic phenolic antioxidants and transformation products in urban and rural indoor dust,” Environmental Pollution., Vol. 221, pp. 227–233, 2017.
W. Wang, A. G. Asimakopoulos, K. O. Abualnaja, A. Covaci, B. Gevao, B. Johnson-Restrepo, T.A. Kumosani, G. Malarvannan, T.B. Minh, H.B. MoonH. Nakata, R.K. Sinha, K. Kannan, “Synthetic phenolic antioxidants and their metabolites in indoor dust from homes and microenvironments,” Environmental Science and Technology, Vol. 50, pp. 428–434, 2016.
D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber, “Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: a national reconnaissance,” Environmental Science and Technology, Vol. 36, pp. 1202–1211, 2002.
M. L. Davi and F. Gnudi, “Phenolic compounds in surface water,” Water Research, Vol. 33, pp. 3213–3219, 1999.
M. A. Soliman, J. A. Pedersen, H. Park, A. Castaneda-Jimenez, M. K. Stenstrom, and I. H. Suffet, “Human pharmaceuticals, antioxidants, and plasticizers in wastewater treatment plant and water reclamation plant effluents,” Water Environment Research, Vol. 79, pp. 156–167, 2007.
W. Wang and K. Kannan”, Inventory, loading and discharge of synthetic phenolic antioxidants and their metabolites in wastewater treatment plants”, Water Research, 129:413–418, 2018.
R. Z. Liu, S. J. Song, Y. F. Lin, T. Ruan, and G. B. Jiang, “Occurrence of synthetic phenolic antioxidants and major metabolites in municipal sewage sludge in China,” Environmental Science and Technology, Vol. 49, pp. 2073–2080, 2015.
R. Liu, T. Ruan, S. Song, Y. Lin, and G. Jiang, “Determination of synthetic phenolic antioxidants and relative metabolites in sewage treatment plant and recipient river by high performance liquid chromatography electrospray tandem mass spectrometry,” Journal of Chromatography A, Vol. 1381, pp. 13–21, 2015.
R. Rodil, J. B. Quintana, and R. Cela, “Oxidation of synthetic phenolic antioxidants during water chlorination,” Journal of Hazardous Materials, Vol. 199–200, pp. 73–81, 2012.
R. Liu and S. A. Mabury, “Synthetic phenolic antioxidants in personal care products in Toronto, Canada: occurrence, human exposure, and discharge via greywater,” Environmental Science and Technology, Vol. 53, pp. 13440–13448, 2019.
T. Delanghe, J. Huyghe, S. Lee, D. Priem, S. Van Coillie, B. Gilbert, S. M. Choi, P. Vandenabeele, A. Degterev, G. D. Cuny, Y. Dondelinger & M. J. M. Bertrand, “Antioxidant and food additive BHA prevents TNF cytotoxicity by acting as a direct RIPK1 inhibitor,” Cell Death and Disease, Vol. 12, p. 699, 2021.
G. M. Williams, M. J. Iatropoulos, and J. Whysner, “Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives,” Food and Chemical Toxicology, Vol. 37, pp. 1027–1038, 1999.
H. B. Conacher, F. Iverson, P. Y. Lau, and B. D. Page, “Levels of BHA and BHT in human and animal adipose tissue: interspecies extrapolation,” Food and Chemical Toxicology, Vol. 24, pp. 1159–1162, 1986.
B. Du, Y. Zhang, J. C. W. Lam, S. Pan, Y. Huang, B. Chen, “Prevalence, biotransformation, and maternal transfer of synthetic phenolic antioxidants in pregnant women from South China,” Environmental Science and Technology, Vol. 53, pp. 13959–13969, 2019.
Y. Zhang, B. Du, J. Ge, L. Y. Liu, and L. Zeng, “Cooccurrence of and infant exposure to multiple common and unusual phenolic antioxidants in human breast milk,” Environmental Science & Technology Letters, Vol. 7, pp. 206–212, 2020.
W. Wang and K. Kannan, “Quantitative identification of and exposure to synthetic phenolic antioxidants, including butylated hydroxytoluene, in urine,” Environment International, Vol. 128, pp. 24–29, 2019.
R. Liu and S. A. Mabury, “Unexpectedly high concentrations of 2,4-ditert-butylphenol in human urine,” Environmental Pollution, Vol. 252, pp. 1423–1428, 2019.
C. Li, X. Y. Cui, Y. Chen, C. Y. Liao, and L. Q. Ma, “Synthetic phenolic antioxidants and their major metabolites in human fingernail,” Environmental Research, Vol. 169, pp. 308–314, 2019.
R. Z. Liu and S. A. Mabury, “Synthetic phenolic antioxidants and transformation products in human sera from United States donors,” Environmental Science & Technology Letters, Vol. 5, pp. 419–423, 2018.
F. Iverson, “In vivo studies on butylated hydroxyanisole,” Food and Chemical Toxicology, Vol. 37, pp. 993–997, 1999.
X. X. Yang, Z. D. Sun, W. Y. Wang, Q. F. Zhou, G. Q. Shi, F. S. Wei, G. Jiang, “Developmental toxicity of synthetic phenolic antioxidants to the early life stage of zebrafish,” Science of the Total Environment, Vol. 643, pp. 559–568, 2018.
A. Baran, S. Yildirim, A. Ghosigharehaghaji, İ. Bolat, E. Sulukan, and S. B. Ceyhun, “An approach to evaluating the potential teratogenicity and perturbations in hepatic lipid metabolism in zebrafish embryo (Danio rerio),” Human & Experimental Toxicology, Vol. 40, pp. 425–438, 2021.
S. H. Jeong, B. Y. Kim, H. Kang, H. Ku, and J. H. Cho, “Effects of butylated hydroxyanisole on the development and functions of reproductive system in rats,” Toxicology, Vol. 208, pp. 49–62, 2005
Z. Sun, X. Yang, Q. S. Liu, C. Li, Q. Zhou, H. Fiedler, “Butylated hydroxyanisole isomers induce distinct adipogenesis in 3T3-L1 cells,” Journal of Hazardous Materials, Vol. 379, p. 120794, 2019.
Z. Sun, Z. Tang, X. Yang, Q. S. Liu, J. Zhang, Q. Zhou, “3-tert-Butyl-4-hydroxyanisole impairs hepatic lipid metabolism in male mice fed with a high-fat diet,” Environmental Science & Technology, Vol. 56, pp. 3204–3213, 2022.
Z. Sun, Z. Tang, X. Yang, Q. S. Liu, Y. Liang, H. Fiedle, “Perturbation of 3-tert-butyl-4-hydroxyanisole in adipogenesis of male mice with normal and high fat diets,” Science of the Total Environment, Vol. 703, p. 135608, 2020.
K. Morimoto, T. Takahashi, K. Okudaira, T. Iio, Y. Saito, and A. Takahashi, “Dose-response study on covalent binding to forestomach protein from male F344 rats following oral administration of [14C]3-BHA,” Carcinogenesis, Vol. 13, pp. 1663–1666, 1992.
K. Morimoto, K. Tsuji, T. Iio, N. Miyata, A. Uchida, R. Osawa, H. Kitsutaka , A. Takahashi, “DNA damage in forestomach epithelium from male F344 rats following oral administration of tert-butylquinone, one of the forestomach metabolites of 3-BHA,” Carcinogenesis, Vol. 12, pp. 703–708, 1991. ,
L. K. Lam and P. Garg, “Tumorigenicity of di-tert-butyl-substituted hydroquinone and hydroxyanisoles in the forestomach of Syrian golden hamsters,” Carcinogenesis, Vol. 12, pp. 1341–1344, 1991.
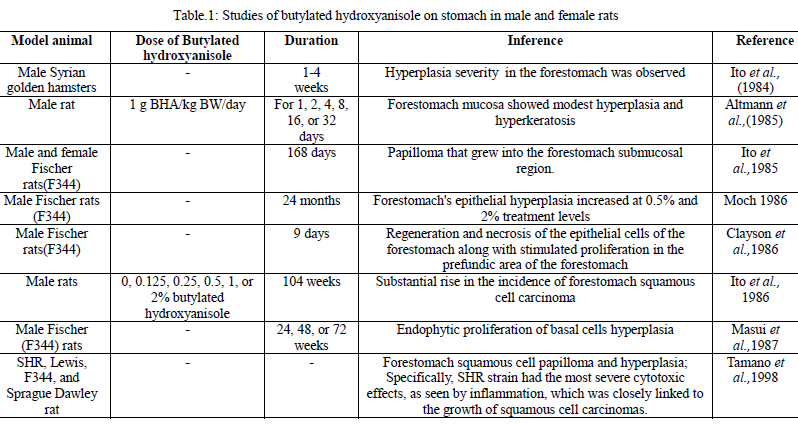
H. J. Altmann, P. W. Wester, G. Matthiaschk, W. Grunow, and C. A. van der Heijden, “Induction of early lesions in the forestomach of rats by 3-tert-butyl-4-hydroxyanisole (BHA),” Food and Chemical Toxicology, Vol. 23, pp. 723–731, 1985.
N. Ito, S. Fukushima, A. Hagiwara, M. Shibata, and T. Ogiso, “Carcinogenicity of butylated hydroxyanisole in F344 rats,” Journal of the National Cancer Institute, Vol. 70, pp. 343–352, 1983.
S. Park, J. Y. Lee, W. Lim, S. You, and G. Song, “Butylated hydroxyanisole exerts neurotoxic effects by promoting cytosolic calcium accumulation and endoplasmic reticulum stress in astrocytes,” Journal of Agricultural and Food Chemistry, Vol. 67, pp. 9618–9629, 2019.
J. Ham, W. Lim, S. You, and G. Song, “Butylated hydroxyanisole induces testicular dysfunction in mouse testis cells by dysregulating calcium homeostasis and stimulating endoplasmic reticulum stress,” Science of the Total Environment, Vol. 702, pp. 134775, 2020.
X. X. Yang, W. T. Song, N. Liu, Z. D. Sun, R. R. Liu, Q. S. Liu, Q. Zhou, G. Jiang, “Synthetic phenolic antioxidants cause perturbation in steroidogenesis in vitro and in vivo,” Environmental Science & Technology, Vol. 52, pp. 850–858, 2018.
S. Vandghanooni, A. Forouharmehr, M. Eskandani, A. Barzegari, V. Kafil, S. Kashanian and J. E. N. Dolatabadi, “Cytotoxicity and DNA fragmentation properties of butylated hydroxyanisole,” DNA and Cell Biology, Vol. 32, pp. 98–103, 2013.
V. de Oliveira Pateis, L. Bracht, L. dos Santos Castro, G. B. F. Salla., J. F. Comar, A. V. Parizotto, R. M. Peralta, A. Bracht, “The food additive BHA modifies energy metabolism in the perfused rat liver,” Toxicology Letters, Vol. 299, pp. 191–200, 2018 ,
N. Ito, M. Hirose, Y. Kurata, E. Ikawa, Y. Mera, and S. Fukushima, “Induction of forestomach hyperplasia by crude butylated hydroxyanisole, a mixture of 3-tert and 2-tert isomers, in Syrian golden hamsters is due to 3-tert-butylated hydroxyanisole,” Gan, Vol. 75, no. 6, pp. 471–474, 1984.
H. J. Altmann, P. W. Wester, G. Matthiaschk, W. Grunow, and C. A. Heijden, “Induction of early lesions in the forestomach of rats by 3-tert-butyl-4-hydroxyanisole (BHA),” Food and Chemical Toxicology, Vol. 23, no. 8, pp. 723–731, 1985.
N. Ito, S. Fukushima, and H. Tsuda, “Carcinogenicity and modification of the carcinogenic response by BHA, BHT, and other antioxidants,” Critical Reviews in Toxicology, Vol. 15, no. 2, pp. 109–15, 1985.
R. W. Moch, “Pathology of BHA- and BHT-induced lesions,” Food and Chemical Toxicology: an international journal published for the British Industrial Biological Research Association, Vol. 24, no. 10–11, pp. 1167–1169, 1986.
D. B. Clayson, F. Iverson, E. Nera, E. Lok, C. Rogers, C. Rodrigues, D. Page, and K. Karpinski, “Histopathological and radioautographical studies on the forestomach of F344 rats treated with butylated hydroxyanisole and related chemicals,” Food and Chemical Toxicology, Vol. 24, no. 10–11, pp. 1171–1182, 1986.
N. Ito, S. Fukushima, S. Tamano, M. Hirose, and A. Hagiwara, “Dose response in butylated hydroxyanisole induction of forestomach carcinogenesis in F344 rats,” Journal of the National Cancer Institute, Vol. 77, no. 6, pp. 1261–1265, 1986.
T. Masui, M. Asamoto, M. Hirose, S. Fukushima, and N. Ito, “Regression of simple hyperplasia and papillomas and persistence of basal cell hyperplasia in the forestomach of F344 rats treated with butylated hydroxyanisole,” Cancer Research, Vol. 47, no. 19, pp. 5171–5174, 1987.
S. Tamano, M. Hirose, H. Tanaka, A. Hagiwara, and T. Shirai, “Variation in susceptibility to the induction of forestomach tumours by butylated hydroxyanisole among rats of different strains,” Food and Chemical Toxicology: an international journal published for the British Industrial Biological Research Association., Vol. 36, no. 4, pp. 299–304, 1998.
S. M. Ford, J. B. Hook, and J. T. Bond, “The effects of butylated hydroxyanisole and butylated hydroxytoluene on renal function in the rat. I. Effects on fluid and electrolyte excretion,” Food and Cosmetics Toxicology., Vol. 18, no. 1, pp. 15–20, 1980.
S. M. Ford, J. B. Hook, and J. T. Bond, “The effects of butylated hydroxyanisole and butylated hydroxytoluene on renal function in the rat. II. Effects on organic acid and base transport,” Food and Cosmetics Toxicology, Vol. 18, no. 1, pp. 21–26, 1980.
C. L. Miranda, M. C. Henderson, and D. R. Buhler, “Dietary butylated hydroxyanisole reduces covalent binding of acetaminophen to mouse tissue proteins in vivo,” Toxicology Letters, Vol. 25, no. 1, pp. 89–93, 1985.
L. I. McLellan, D. J. Harrison, and J. D. Hayes, “Modulation of glutathione S-transferases and glutathione peroxidase by the anticarcinogen butylated hydroxyanisole in murine extrahepatic organs,” Carcinogenesis, Vol. 13, no. 12, pp. 2255–2261, 1992.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Yamini Makarwar, Varsha Dhurvey

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors contributing to this journal agree to publish their articles under the Creative Commons Attribution 4.0 International License, allowing third parties to share their work (copy, distribute, transmit) and to adapt it, under the condition that the authors are given credit and that in the event of reuse or distribution, the terms of this license are made clear.






